 The Centers for Disease Control and Prevention estimate that foodborne infections cause an estimated 76 million cases of illness and 323,000 hospitalizations annually. Patient related costs for treatment of bacterial infections alone are $ 6.7 billion.
The Centers for Disease Control and Prevention estimate that foodborne infections cause an estimated 76 million cases of illness and 323,000 hospitalizations annually. Patient related costs for treatment of bacterial infections alone are $ 6.7 billion.
The most severe illnesses are caused by five main pathogens resulting in 3.5 million infections, 33,000 hospitalizations and 1,600 deaths each year:
The illnesses and deaths caused by the above pathogens ARE COMPLETELY AND ENTIRELY PREVENTABLE by the proper irradiation of the food.
Lexatronics, LLC is a Michigan based company that has a patent pending for the use of low energy x-rays for the irradiation of foodstuffs. In this case, low energy is defined as an x-ray energy spectrum for which the maximum energy is 250 kVp or less. The actual maximum energy used is dependent upon the product being irradiated and the level of penetration that is necessary.
Our specialized process would allow a food processor to install one of our machines in their own plant and irradiate foodstuffs in-line with their other processes. For example, our machines could be placed in the current product line as the last step prior to packaging. Our approach provides for the custom design of the machine for very specific products and product shapes. We have a prototype machine (Rainbow I) built and currently in use in Wixom, Michigan.
This process can be used to treat a variety of foods ranging from red meats and poultry to fruits, vegetables and eggs, to fruit juices or any of the other approved items to destroy food borne pathogens and extend shelf life. Our process would also be ideal for treating ready-to-eat items such as lunchmeats or deli foods to eliminate Listeria.
We have used our process to successfully irradiate:
In all cases we were able to irradiate the product without adverse effects to the product and increased shelf life significantly over non-irradiated product.
We have been able to
We differ from our competitors in three very important ways:
First, our design allows for the safe and easy use in presently existing food processing plants thereby eliminating the expense of transportation to and from central facilities.
Secondly, our process is designed to work on small packages or small amounts, which maximizes uniform dosing, thereby eliminating the burning and off-taste often associated with irradiated foods.
Third, our process is inherently safe because of its ability to be turned off at the end of the day and it does not require extensive shielding such as that necessary for high energy x-rays or Gamma sources such as Co-60.
We are currently the only company that has been able to successfully irradiate fresh orange juice and fresh grapefruit juice without changing the color or taste. This process is able to provide fresh squeezed taste with a refrigerated shelf life of at least 30 days.
Our processed juice was evaluated by food scientists at the University of Florida Extension Service and absolutely no difference in taste could be detected between the treated juice and the control sample. Based on our experience with our prototype machine, we believe that our design for a commercial juice machine will deliver 100 gallons per minute of properly irradiated juice.
Microbial inactivation by all types of ionizing radiation is thought to happen through two main mechanisms: direct interaction of the radiation with cell components and indirect action from radiolytic products, such as the water radicals H+, OH-, and e-aq. The primary target of ionizing radiation appears to be chromosomal DNA, although effects on the cytoplasmic membrane may also play a role. Changes to chromosomal DNA and/or cytoplasmic membrane can cause microbial inactivation or growth inhibition. Many studies have shown that ions, excited atoms and molecules generated during irradiation have no toxic effect on humans.
Verification of the “Proof of Principle” of using low energy x-rays to kill foodborne pathogens was necessary since previous research using photons (X-rays and Gammas) were performed at the MeV level. The pathogen used as a test subject was Escherichia coli ATTC #35421. The Escherichia coli analysis was performed by Microbiological Associates, Inc., 37428 Hills Tech Drive, Farmington Hills, MI 48331.
This E-coli was chosen for two important reasons: 1) it was a surrogate pathogen and not active on human beings, and 2) it was one of the more vigorous E-coli that could be heavily vitalized to provide a strong target for our process.
A loop of the stock solution was transferred to several plates of Endo Agar and allowed to incubate at 35-37 Centigrade for 24 hours. An inspection was made to insure that all CFU’s were compatible with that of the Coliform bacteria. Several selected typical coliform colonies were then transferred to six separate tubes of MUG media and the fluorescence characteristics confirmed for Escherichia coli.
Aliquots of the characteristic CFU’s were then transferred to an additional bottle of Lactose broth, incubated for 48 hours and the final solution designated “Stock Std.”
As a final confirmation, a loop full of the stock standard was plated on a dish of M Endo Agar as well as MUG medium to confirm the presence of Coliform as Escherichia coli. colonies.
The carrier solution used to suspend the E-coli during irradiation was distilled water. The solution used to dilute the original Stock Std. for each sample was a phosphate buffer.
Initial stock solutions containing approximately 1 million organisms/ml. were made and placed in 125 ml. HDPE bottles such as those shown in Figure 1. 
A magnetic stir was placed in each bottle to ensure complete mixing during the irradiation. The small white square seen on the outside of the bottle is a Thermo Luminescent Dosimeter (TLD_ used to measure deposited dose. These were placed on the outside of the bottle to measure the external dose field and inside the bottle to measure absorbed dose in the fluid. The preferred bottle for irradiation is PET. PET bottles can be much thinner and absorb less radiation than HDPE and have also been approved for irradiation and for the containment of orange juice.
The bottles were then placed in the irradiator on a stir and rotate platform one at a time as shown in Figure 2.
 Each sample consisted of a 125 ml. solution provided by Microbiological Associates, Inc. Each sample was refrigerated at 4 degrees C until transferred to the irradiation location. Each sample was stored at the irradiation location in a refrigerator at 4 degrees C until placed in the irradiation chamber and given various doses of low energy x-rays and then returned to the refrigerator until transferred for analysis. During transit the samples were also stored in a cooler with ice packs. Controls were also used in parallel to each sample where the matching control was treated exactly as the irradiated sample except for the irradiation.
Each sample consisted of a 125 ml. solution provided by Microbiological Associates, Inc. Each sample was refrigerated at 4 degrees C until transferred to the irradiation location. Each sample was stored at the irradiation location in a refrigerator at 4 degrees C until placed in the irradiation chamber and given various doses of low energy x-rays and then returned to the refrigerator until transferred for analysis. During transit the samples were also stored in a cooler with ice packs. Controls were also used in parallel to each sample where the matching control was treated exactly as the irradiated sample except for the irradiation.
The samples were then returned to Microbiological Associates, Inc. and a heterotrophic plate count (HPC) procedure used to determine the number of living organisms/ml. Every sample was run in triplicate with three plates for each of three dilutions created. The plates were incubated for 48 hours and then the total plate count was reported.
Figure 3 shows the average result for a single representative series of samples. This figure demonstrates the reduction of the E-coli population over a range of eight logs. FDA regulations require that any method used to reduce foodborne pathogens must be capable of reducing the pathogen population by five logs. Our method clearly demonstrates this capability to meet and exceed the five log reduction requirement.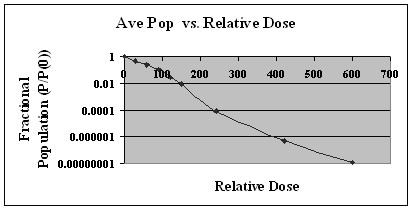
Log Reduction vs. Dose
Figure 3. Fractional Population (Post-Irradiation Population/Initial Pre-Irradiation Population) vs. Relative Dose.
Figure 4 shows an image of two plates, one a control plate at a 1/10,000 dilution (Sample BK-53, Population 1,041,667 organisms/ml.) and a post irradiation plate at a 1/1 ml. dilution (Sample BK-54, Population 10 organisms/ml.).
|
|
Once the ability to reduce pathogen population by five logs was demonstrated it was then necessary to once again test the effect on taste by irradiating a sample product for a period of time represented by the five log reduction. For this step two juices were chosen; fresh squeezed orange juice and fresh squeezed apple cider. Multiple controls and irradiated samples were produced and set up in a blind taste test with people outside Lexatronics, LLC. In all cases, those participating in the taste tests were unable to determine which samples had been irradiated when given three samples where one of the samples was a control and the other two irradiated or with the numbers reversed. As mentioned previously, our processed juice was evaluated by food scientists at the University of Florida Extension Service and absolutely no difference in taste could be detected between the treated juice and the control sample.
In summary, we have demonstrated the ability of low energy x-rays to eradicate foodborne pathogens without adversely affecting the taste for several food products. In addition, this process is capable of significantly extending the shelf life of the food product irradiated. We have a prototype machine in use which is economical, portable, safe and infinitely configurable for a multitude of applications.
Rayfresh Foods has developed an economical way to irradiate food products within the scheme of a continuous process. This in-line system helps in the quest for food safety. Our by-products are safe, healthy foods, and extended shelf life with no discernable change in taste and texture. By passing light through a product using x-rays, we can only add to a processors already clean and safe practices. Although no one thing can eliminate all the risks associated with food processing, Rayfresh Foods Rainbow Process will move us closer to safe tables.